Background: Acute painful vaso-occlusive crises (VOCs), the hallmark of sickle cell disease (SCD), are associated with chronic and potentially life-threatening complications. The SOLACE-adults study (NCT03264989) interim analysis (cutoff date: August 1, 2020) demonstrated the long-term pharmacokinetic (PK) and pharmacodynamic (PD) properties, potential sustained efficacy (VOC reduction), and long-term safety of crizanlizumab 5 mg/kg and 7.5 mg/kg during ≥12 months' (mo) treatment in >80% of patients (pts) with SCD [Kanter J et al . Blood advances 2023]. Here we report the updated PK/PD, including ex vivo P-selectin inhibition, safety, and efficacy results from the final interim analysis of this study (cutoff date: June 1, 2022) for all pts who received crizanlizumab 5 mg/kg (~3.5 years [y]) and 7.5 mg/kg (~3 y).
Methods: This is a phase 2, multicenter, open-label study in pts with SCD, aged 16 to 70 y, who had experienced ≥1 VOC (defined as pain crises and complicated SCD crises such as acute chest syndrome, priapism and hepatic or splenic sequestration) in the 12 mo prior to screening. Pts were enrolled into 5 mg/kg and then 7.5 mg/kg groups sequentially and received crizanlizumab by intravenous infusion over 30 minutes on day 1, day 15, and every 4 weeks (wk) thereafter. Pts receiving hydroxyurea/hydroxycarbamide (HU/HC) and/or erythropoietin-stimulating agents for at least 6 mo prior to screening were allowed to continue the same dose and schedule during the study.
Results: Overall, 57 pts were enrolled: 45 received crizanlizumab 5 mg/kg (median age, 29 [IQR 22, 39] y) for a median of 191 (IQR 98, 210) wk, and 12 received 7.5 mg/kg (median age, 21 [IQR 18, 41] y) for a median of 167 (IQR 120,182) wk. At cutoff, 22 pts (49%) in 5 mg/kg group and 6 pts (50%) in 7.5 mg/kg group discontinued the treatment, primarily because of physicians' (5 mg/kg, n=6; 7.5 mg/kg, n=4) and pts' decisions (5 mg/kg, n=9).
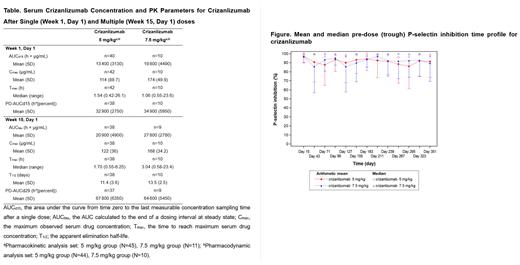
For both doses, serum crizanlizumab concentrations increased to nearly maximum levels (C max) at the end of the 30-minute infusion and remained steady for 6 hours after infusion. For each dose, C max at wk 1 and 15 was similar ( Table), indicating no significant accumulation. Crizanlizumab exposure increased almost dose proportionally from 5 mg/kg to 7.5 mg/kg. P-selectin inhibition was nearly complete throughout the dosing interval for both doses at steady state (5 mg/kg, 90% to 98%; 7.5 mg/kg, 86% to 96%), with consistent and stable pre-dose inhibition throughout the study ( Figure).
All pts reported ≥1 adverse events (AEs) in both dose groups. The most common AEs were pyrexia (5 mg/kg, 14 [31%]; 7.5 mg/kg, 3 [25%]), headache and hypokalemia (5 mg/kg, 12 [27%]; 7.5 mg/kg, 2 [17%], each). Grade ≥3 AEs occurred in 27 pts (60%) in the 5 mg/kg group and in 6 pts (50%) in the 7.5 mg/kg group. At least one serious AE was reported in 22 pts (49%) in the 5 mg/kg group and 5 pts (42%) in the 7.5 mg/kg group; 1 was drug related in 5 mg/kg group (none in the 7.5mg/kg group). One pt in each of the 5 mg/kg (2%) and 7.5 mg/kg (8%) groups discontinued treatment because of drug-related AEs. One pt in each group died while on treatment, but the death was not considered related to crizanlizumab. Two pts in the 5 mg/kg group had severe crizanlizumab-related infusion-related reactions (IRR) (grade 2 pain [n=1], grade 3 IRR [n=1]), which resolved with treatment. No grade ≥3 treatment-related infections or bleeding events were reported. No pts developed antibodies against crizanlizumab.
The median (IQR) annualized rate of VOCs leading to a healthcare visit in the 5 mg/kg group was 4 (1, 7) at baseline and 2.75 (1.03, 5.32) on treatment; absolute change from baseline, -0.76 (-2.94, 2.01). In the 7.5 mg/kg group, the corresponding rates were 2 (1, 4.5) and 1.09 (0.30, 3.36), -0.91 (-1.06, -0.50). Eight pts (18%) in the 5 mg/kg group and 2 pts (17%) in the 7.5 mg/kg group were VOC free during the entire treatment period.
Conclusions: These long-term results for ~3.5 y in 5 mg/kg and ~3 y in 7.5 mg/kg groups demonstrate that serum crizanlizumab concentrations rose to a near maximum level shortly after infusion and remained steady 6 hours after infusion for both doses. Crizanlizumab with or without HU/HC was safe with no new/unexpected safety concerns and no apparent differences between the two doses in the frequency and severity of AEs. Consistent with previously reported results from SUSTAIN study, crizanlizumab reduced the annualized rate of VOCs leading to a healthcare visit from baseline.
Disclosures
Kanter:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; HRSA: Research Funding; NHLBI: Research Funding; CDC: Research Funding; Novo Nordisk: Research Funding; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BEAM: Consultancy, Research Funding; Takeda: Research Funding; Glycomimetics: Membership on an entity's Board of Directors or advisory committees; Fulcurm: Consultancy; ECOR-1: Consultancy; Guidepoint Global: Honoraria; Vertex: Consultancy; Chiesi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bausch: Consultancy; Austin Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Watkins, Lourie, Roll & Chance: Consultancy; National Alliance of Sickle Cell Centers: Other: President. Manwani:Novartis, Pfizer, Novo Nordisk, Editas, GBT: Consultancy. Kutlar:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GBT/Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forma/Novo-Nordisk, Akira Bio: Research Funding; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Vertex: Other: Event adjudication committee (EAC) Chair. Shah:Bluebird bio: Consultancy; Vertex: Consultancy; Alexion Pharmaceuticals: Speakers Bureau; Global Blood Therapeutics/Pfizer: Consultancy, Research Funding, Speakers Bureau; Agios Pharmaceuticals: Consultancy; Forma: Consultancy. Keefe:Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA: Current Employment, Other: Eligible to receive stocks. Madhamshetty:Novartis Healthcare Private Limited, Hyderabad, India: Current Employment. Reshetnyak:Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA: Current Employment. Mendonza:Novartis Institutes for BioMedical Research, Cambridge, Massachusetts: Current Employment, Other: Eligible to receive stocks. Liles:Abbvie: Other: Clinical trial activity (Principal investigator or sub-investigator); Annexon Biosciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Alpine Immune Sciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Astex Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); Baxalta: Other: Clinical trial activity (Principal investigator or sub-investigator); BeiGene: Other: Clinical trial activity (Principal investigator or sub-investigator); Bioverativ: Other: Clinical trial activity (Principal investigator or sub-investigator); CSL Behring: Other: Clinical trial activity (Principal investigator or sub-investigator); Celgene: Other: Clinical trial activity (Principal investigator or sub-investigator); Delta-Fly Pharma: Other: Clinical trial activity (Principal investigator or sub-investigator); Exact Sciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Forma Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Global Blood Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Immunovant: Other: Clinical trial activity (Principal investigator or sub-investigator); Incyte: Other: Clinical trial activity (Principal investigator or sub-investigator); Janssen Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); NeoImmuneTech: Other: Clinical trial activity (Principal investigator or sub-investigator); Novartis: Other: Clinical trial activity (Principal investigator or sub-investigator); Novo Nordisk: Other: Clinical trial activity (Principal investigator or sub-investigator); Partner Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Pharm-Olam: Other: Clinical trial activity (Principal investigator or sub-investigator); Principia Biopharma: Other: Clinical trial activity (Principal investigator or sub-investigator); Salix Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); Sanofi-Aventis: Other: Clinical trial activity (Principal investigator or sub-investigator); Takeda: Other: Clinical trial activity (Principal investigator or sub-investigator); Vifor Pharma: Other: Clinical trial activity (Principal investigator or sub-investigator).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal